Cerilliant® Certified Reference Materials
Empowered by Legacy, Driven by Accuracy
Power your results with Cerilliant® high-quality certified reference materials, expertly crafted for forensic, clinical, pharmaceutical, nutraceutical and environmental testing and research.
ISO 9001:2015
ISO/IEC 17025:2017
ISO 17034:2016
Accuracy Matters
Since 1980, Cerilliant® Certified Reference Materials (CRMs) have set the standard for trust and excellence, combining industry-leading quality and comprehensive selection with data-inclusive certificates of analysis (COAs). Empowered by our legacy of high quality and reliability, we are driven by accuracy and innovation to ensure your confidence and success at every step. Results are only as accurate as your reference!
45+
Years of Excellence and Experience
150+
Passionate and Curious Scientists & Support Staff
33%
Staff with 10+ years of Cerilliant® Service
2300+
CRMs to Drive Accuracy for Your Analytical Results
Your Benefits
Simplify your analytical process with trusted standards, accredited quality, and data-driven performance — all backed by Cerilliant’s proven expertise and commitment to precision.

Convenient Snap-N-Shoot® & Certified Spiking Solutions®
ISO/IEC 17025, ISO 17034 Accredited
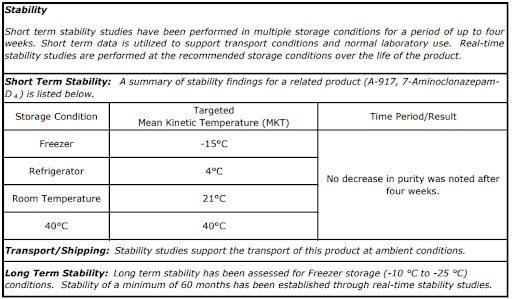
Data-Driven Expiry and Storage Requirements
Comprehensive Certificates of Analysis (COAs)
Custom Standard Services
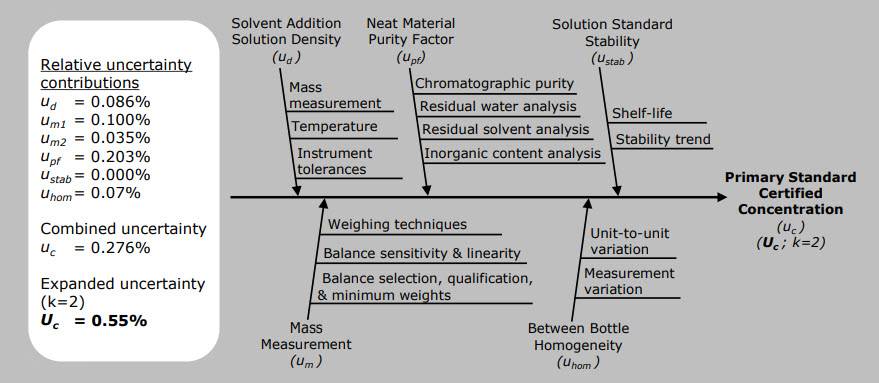
Understanding
Your Cerilliant® Certificate of Analysis
Products
Cerilliant® Certified Reference Materials (CRMs) include native and stable-isotope labeled parent, metabolites, and related compounds for clinical, forensic, pharmaceutical, nutraceutical, environmental and other testing and research applications.
Illicit Drug, Pharmaceutical, & Alcohol Standards
Stimulants, depressants, opioids, hallucinogens, cannabinoids, inhalants, synthetic drugs, and others for toxicological testing applications.
Biomarker & Metabolite
Standards
Standards
Hormones, hydroxyvitamin D, amino acid metabolites, catecholamines, and other endogenous biomarkers.
Vitamins & Other
Natural Products
Natural Products
Water- and fat-soluble vitamin, phytochemicals, protein, peptide, and lipid standards for various chromatography & mass spectrometry testing applications.